
Chemistry, 08.04.2020 19:25 angelaisthebest1700
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total pressure of the gases above an equilibrium mixture if, at equilibrium, PHI = 0.010 × PH2 S?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

You know the right answer?
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total p...
Questions




Mathematics, 05.09.2019 19:30

History, 05.09.2019 19:30

Mathematics, 05.09.2019 19:30



Biology, 05.09.2019 19:30




Advanced Placement (AP), 05.09.2019 19:30


Social Studies, 05.09.2019 19:30





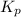
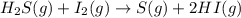
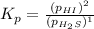
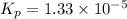
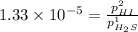
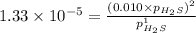
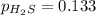
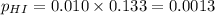
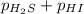 = 0.133+0.0013 = 0.1343 atm
= 0.133+0.0013 = 0.1343 atm

