
Chemistry, 08.04.2020 20:07 Darkknighta26
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the reaction mechanism consist of only a single bimolecular step? 1. yes, it must 2. it might, but you cannot tell from the information given. 3. no, it cannot

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the r...
Questions


Social Studies, 31.01.2020 21:57

Biology, 31.01.2020 21:57

Physics, 31.01.2020 21:57

English, 31.01.2020 21:57




Chemistry, 31.01.2020 21:57



Computers and Technology, 31.01.2020 21:57




World Languages, 31.01.2020 21:57


Social Studies, 31.01.2020 21:57

SAT, 31.01.2020 21:57

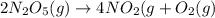
![Rate=k[N_2O_5]^1](/tpl/images/0589/8713/ae447.png)
 , it is a complex reaction.
, it is a complex reaction.

