
Chemistry, 08.04.2020 21:35 charlesiarenee0
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a reaction quotient of 525. Which direction will the system shift to?
The equilibrium will shift to the left to favor the reactants.
The equilibrium will shift to the right to favor the products.
The equilibrium will not shift in any direction.
The equilibrium will shift to the forward reaction.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

You know the right answer?
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a react...
Questions








Advanced Placement (AP), 08.12.2020 22:20

Mathematics, 08.12.2020 22:20



Mathematics, 08.12.2020 22:20

Mathematics, 08.12.2020 22:20

Mathematics, 08.12.2020 22:20

Computers and Technology, 08.12.2020 22:20


Mathematics, 08.12.2020 22:20



Mathematics, 08.12.2020 22:20

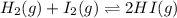
![Q=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0590/2867/236ed.png)
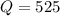

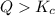 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.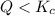 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.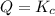 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

