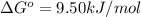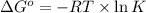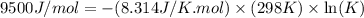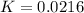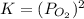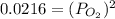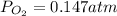Chemistry, 08.04.2020 21:39 audjwood67
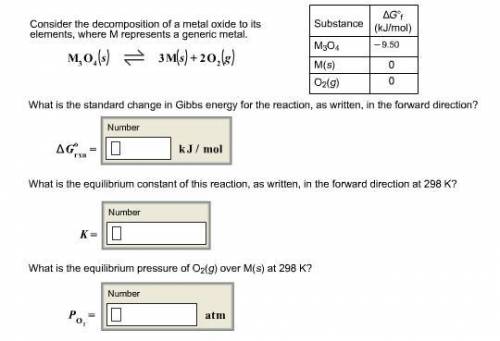
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3 O 4 ( s ) − ⇀ ↽ − 3 M ( s ) + 2 O 2 ( g ) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? Δ G ∘ rxn = kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K = What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
You know the right answer?
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3...
Questions



Mathematics, 23.05.2021 02:30



Social Studies, 23.05.2021 02:30




History, 23.05.2021 02:30




Mathematics, 23.05.2021 02:30

Mathematics, 23.05.2021 02:30


English, 23.05.2021 02:30


Mathematics, 23.05.2021 02:30

Chemistry, 23.05.2021 02:30

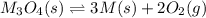
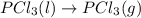
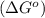 .
.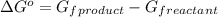
![\Delta G^o=[n_{M(s)}\times \Delta G^0_{(M(s))}+n_{O_2(g)}\times \Delta G^0_{(O_2(g))}]-[n_{M_3O_4(s)}\times \Delta G^0_{(M_3O_4(s))}]](/tpl/images/0590/3061/56a28.png)
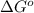 = Gibbs energy of reaction = ?
= Gibbs energy of reaction = ?![\Delta G^o=[3mole\times (0kJ/mol)+2mole\times (0kJ/mol)]-[1mole\times (-9.50kJ/K.mol)]](/tpl/images/0590/3061/a6722.png)