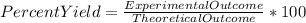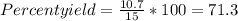Chemistry, 08.04.2020 22:01 GreenHerbz206
When Wolverine’s 10-pound adamantium claws are dissolved in 100 mL of 10 M nitric acid, 10.7 grams of adamantium nitrate are recovered. If we expected 15.0 grams of adamantium nitrate to be recovered in a complete reaction, what was the percent yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
When Wolverine’s 10-pound adamantium claws are dissolved in 100 mL of 10 M nitric acid, 10.7 grams o...
Questions

Biology, 04.10.2021 16:50



Biology, 04.10.2021 16:50

Mathematics, 04.10.2021 16:50


Social Studies, 04.10.2021 16:50

Health, 04.10.2021 16:50

Biology, 04.10.2021 16:50

History, 04.10.2021 16:50



Physics, 04.10.2021 16:50



Advanced Placement (AP), 04.10.2021 16:50


Geography, 04.10.2021 16:50

History, 04.10.2021 16:50

Mathematics, 04.10.2021 16:50