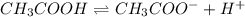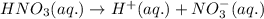Chemistry, 08.04.2020 21:58 rchapman414
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means that the substance is extremely reactive. The term "weak electrolyte" means that the substance is inert. A weak acid solution consists of mostly nonionized acid molecules. A strong acid solution consists of only partially ionized acid molecules.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means tha...
Questions


English, 27.09.2019 02:00






Computers and Technology, 27.09.2019 02:00


History, 27.09.2019 02:00


Biology, 27.09.2019 02:00






Biology, 27.09.2019 02:00


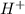 ions when dissolved in water. Thus most of molecules remain unionized in solutions.
ions when dissolved in water. Thus most of molecules remain unionized in solutions.