Chemistry, 09.04.2020 00:19 jdkrisdaimcc11
A student prepares a aqueous solution of trimethylacetic acid . Calculate the fraction of trimethylacetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
A student prepares a aqueous solution of trimethylacetic acid . Calculate the fraction of trimethyla...
Questions

Social Studies, 19.02.2020 08:03





Mathematics, 19.02.2020 08:07






Social Studies, 19.02.2020 08:13

Social Studies, 19.02.2020 08:14

History, 19.02.2020 08:14

Chemistry, 19.02.2020 08:14


Chemistry, 19.02.2020 08:14

Mathematics, 19.02.2020 08:14


Biology, 19.02.2020 08:15

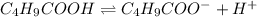
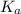 for above equation follows:
for above equation follows:![K_a=\frac{[C_4H_9COO^-][H^+]}{[C_4H_9COOH]}](/tpl/images/0590/8124/fb242.png)
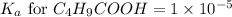
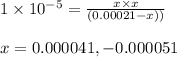
![\text{Percent of dissociation}=\frac{[H^+]}{[C_4H_9COOH]}\times 100](/tpl/images/0590/8124/75101.png)