
Chemistry, 09.04.2020 00:57 ChaseRussell24
A proposed mechanism for the reaction Cl2(g) + CHCl3(g) HCl(g) + CCl4(g) in the atmosphere is Step 1: Cl2(g) 2 Cl(g) (very fast, reversible) Step 2: Cl(g) + CHCl3(g) HCl(g) + CCl3(g) (slow) Step 3: Cl(g) + CCl3(g) CCl4(g) (fast) What is the rate law for the overall reaction?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1

Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2

You know the right answer?
A proposed mechanism for the reaction Cl2(g) + CHCl3(g) HCl(g) + CCl4(g) in the atmosphere is Step...
Questions

Biology, 21.01.2021 19:30





Mathematics, 21.01.2021 19:30




Mathematics, 21.01.2021 19:30

Physics, 21.01.2021 19:30

Mathematics, 21.01.2021 19:30


Chemistry, 21.01.2021 19:30


Chemistry, 21.01.2021 19:30

Physics, 21.01.2021 19:30

Biology, 21.01.2021 19:30


![\text{Rate}=k[Cl_2]^{1/2}[CCl_4][CHCl_3][CCl_3]^{-1}](/tpl/images/0590/9105/bc5d3.png)
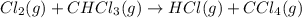
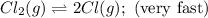
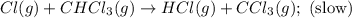
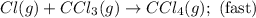
![\text{Rate}=K_2[Cl][CHCl_3]](/tpl/images/0590/9105/4079f.png) ......(1)
......(1)![K_1=\frac{[Cl]^2}{[Cl_2]}](/tpl/images/0590/9105/5c3f3.png)
![[Cl]=\sqrt{K_1[Cl_2]}](/tpl/images/0590/9105/af496.png)
![K_3=\frac{[CCl_4]}{[Cl][CCl_3]}](/tpl/images/0590/9105/b542e.png)
![[Cl]=\frac{[CCl_4]}{K_3[CCl_3]}](/tpl/images/0590/9105/a0988.png)
![\text{Rate}=K_2\times \sqrt{K_1[Cl_2]}\times \frac{[CCl_4]}{K_3[CCl_3]}\times [CHCl_3]\\\\\text{Rate}=k[Cl_2]^{1/2}[CCl_4][CHCl_3][CCl_3]^{-1}](/tpl/images/0590/9105/79491.png)



