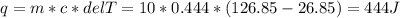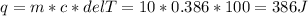Chemistry, 09.04.2020 01:32 Ashleyvasquez2261
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature difference of 100 K).
How much energy, in Joules, was absorbed by the iron block? *
How much energy, in Joules, was absorbed by the copper block? *

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature differe...
Questions


Mathematics, 06.11.2020 14:00

History, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00

English, 06.11.2020 14:00

English, 06.11.2020 14:00

Social Studies, 06.11.2020 14:00

English, 06.11.2020 14:00

English, 06.11.2020 14:00



Law, 06.11.2020 14:00


Social Studies, 06.11.2020 14:00


Mathematics, 06.11.2020 14:00

World Languages, 06.11.2020 14:00