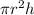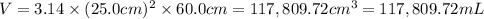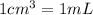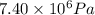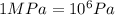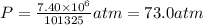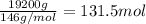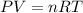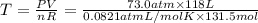Chemistry, 09.04.2020 02:03 naomicervero
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 50.0cm wide and 60.0cm high. The maximum safe pressure inside the vessel has been measured to be 7.40MPa. For a certain reaction the vessel may contain up to 19.2kg of sulfur hexafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas rea...
Questions

History, 27.07.2019 23:00




Social Studies, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00


Biology, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00


Chemistry, 27.07.2019 23:00



Social Studies, 27.07.2019 23:00


Mathematics, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00