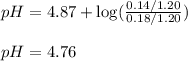Chemistry, 09.04.2020 02:02 astepania0003
A buffer contains 0.17 mol of propionic acid ( C2H5COOH ) and 0.14 mol of sodium propionate (C2H5COONa) in 1.20 L. What is the pH of the buffer after the addition of 0.01 mol of HI

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
A buffer contains 0.17 mol of propionic acid ( C2H5COOH ) and 0.14 mol of sodium propionate (C2H5COO...
Questions


English, 25.04.2021 19:10


Mathematics, 25.04.2021 19:10




Mathematics, 25.04.2021 19:10

Mathematics, 25.04.2021 19:10

Geography, 25.04.2021 19:10


Biology, 25.04.2021 19:10


English, 25.04.2021 19:10

English, 25.04.2021 19:10

Social Studies, 25.04.2021 19:10

Mathematics, 25.04.2021 19:10

English, 25.04.2021 19:10

Chemistry, 25.04.2021 19:10

Mathematics, 25.04.2021 19:10

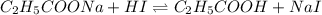
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0591/0789/e4eea.png)
![pH=pK_a+\log(\frac{[C_2H_5COONa]}{[C_2H_5COOH]})](/tpl/images/0591/0789/165ff.png)
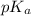 = negative logarithm of acid dissociation constant of propionic acid = 4.87
= negative logarithm of acid dissociation constant of propionic acid = 4.87![[C_2H_5COONa]=\frac{0.14}{1.20}](/tpl/images/0591/0789/fbf02.png)
![[C_2H_5COOH]=\frac{0.18}{1.20}](/tpl/images/0591/0789/e71bf.png)