Chemistry, 09.04.2020 03:05 trevorhenyan51
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutral. a.) [OH−] = 3.8×10−2 Mb.) [OH−] = 1.0×10-7 Mc.) [OH−] = 5.5×10−10 MPlease show the work and how you determine if the solution is neutral, acidic, or basic

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutr...
Questions



English, 18.03.2021 01:30

Biology, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30

History, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Physics, 18.03.2021 01:30


![pH=-log[H_3O^+]](/tpl/images/0591/2031/6e71a.png)
![[H_3O^+][OH^-]=K_w=10^{-14}](/tpl/images/0591/2031/b8614.png) [ at 25°C]
[ at 25°C]![log[H_3O^+]+log[OH^-]=logK_w=log 10^{-14}](/tpl/images/0591/2031/bb8c2.png)
![\Rightarrow - log[H_3O^+]-log[OH^-]=-logK_w=-log 10^{-14}](/tpl/images/0591/2031/a8528.png)
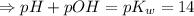
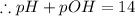
![[OH^-]=3.8\times10^{-2}M](/tpl/images/0591/2031/42d58.png)
![- log[H_3O^+]-log[OH^-]=14}](/tpl/images/0591/2031/0c29a.png)
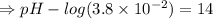
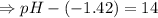
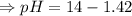
![[OH^-]=1.0\times10^{-7}M](/tpl/images/0591/2031/ac083.png)
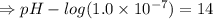
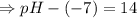
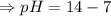
![[OH^-]=5.5\times10^{-10}M](/tpl/images/0591/2031/f6aae.png)