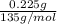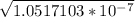Chemistry, 09.04.2020 03:14 ochoachanna
Amphetamine (c9h13n) is a weak base with a pkb of 4.2. calculate the ph of a solution containing an amphetamine concentration of 225 mg>l

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Amphetamine (c9h13n) is a weak base with a pkb of 4.2. calculate the ph of a solution containing an...
Questions


Mathematics, 15.02.2021 20:00




Computers and Technology, 15.02.2021 20:00


Mathematics, 15.02.2021 20:00

Biology, 15.02.2021 20:00


Mathematics, 15.02.2021 20:00


Mathematics, 15.02.2021 20:00

English, 15.02.2021 20:00



English, 15.02.2021 20:00

Mathematics, 15.02.2021 20:00


English, 15.02.2021 20:00

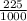 = 0.225 g
= 0.225 g