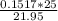Chemistry, 09.04.2020 04:37 Bearboy5957
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indicator are added to an erlenmeyer flask, and the titration experiment is carried out. if the initial buret reading was 0.55 ml, and the buret reading at the endpoint was 22.50 ml, what is the molarity of the unknown acid, ha?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indic...
Questions

SAT, 30.11.2021 19:50



Engineering, 30.11.2021 19:50

English, 30.11.2021 19:50

Mathematics, 30.11.2021 19:50


Arts, 30.11.2021 19:50

Mathematics, 30.11.2021 19:50

English, 30.11.2021 19:50

Mathematics, 30.11.2021 19:50


English, 30.11.2021 19:50

History, 30.11.2021 19:50



Mathematics, 30.11.2021 19:50


Business, 30.11.2021 19:50

Health, 30.11.2021 19:50

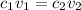 where 1 and 2 are the values for acid and base respectively.
where 1 and 2 are the values for acid and base respectively.