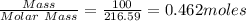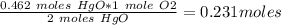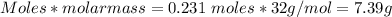Chemistry, 03.02.2020 04:51 michaelwarren8728
In antoine lavoisier’s classic experiment, mercuric oxide is heated in a sealed container. the solid red powder is changed into two products: silver liquid mercury and oxygen gas. if lavoisier heated 100 grams of powdered mercuric oxide to produce 93 grams of liquid mercury, how much oxygen would be released?
a). 7 grams
b). 16 grams
c). 32 grams
d). 93 grams

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
In antoine lavoisier’s classic experiment, mercuric oxide is heated in a sealed container. the solid...
Questions

English, 05.05.2020 05:37









Mathematics, 05.05.2020 05:37


Mathematics, 05.05.2020 05:37

Mathematics, 05.05.2020 05:37