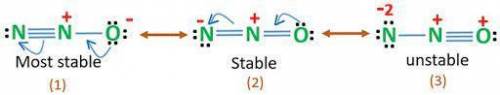
Dinitrogen monoxide has a structural formula of NNO and requires resonance structures in order to draw the Lewis structures of the molecule. Based on formal charge distributions, themostsignificant (stable) resonance structure for this molecule exhibits the order of formal charges for the 1st N, the central N, and the O atoms, respectively, as:
A. 0,+1,-1
B. -1,+1,0
C. -2,+3,-1
D. 0,0,0

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3


Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

You know the right answer?
Dinitrogen monoxide has a structural formula of NNO and requires resonance structures in order to dr...
Questions

Mathematics, 10.09.2021 18:10

History, 10.09.2021 18:10

History, 10.09.2021 18:20

Mathematics, 10.09.2021 18:20

Mathematics, 10.09.2021 18:20


Mathematics, 10.09.2021 18:20

Mathematics, 10.09.2021 18:20


Mathematics, 10.09.2021 18:20



Biology, 10.09.2021 18:20

Mathematics, 10.09.2021 18:20

English, 10.09.2021 18:20

English, 10.09.2021 18:20

Biology, 10.09.2021 18:20



Mathematics, 10.09.2021 18:20