
Chemistry, 20.09.2019 06:20 jonmorton159
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) → p4o6 (s) ∆h = -1640 kj i. heat is absorbed ii. heat is released iii. rxn is exothermic iv. rxn is endothermic v. products have higher enthalpy content than reactants vi. reactants have higher enthalpy content than products

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) →...
Questions

English, 19.08.2019 21:50

Mathematics, 19.08.2019 21:50

Mathematics, 19.08.2019 21:50


Mathematics, 19.08.2019 21:50

History, 19.08.2019 21:50



Mathematics, 19.08.2019 21:50


World Languages, 19.08.2019 21:50



Health, 19.08.2019 21:50


Mathematics, 19.08.2019 21:50

Social Studies, 19.08.2019 21:50

Social Studies, 19.08.2019 21:50

English, 19.08.2019 21:50

Mathematics, 19.08.2019 21:50

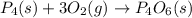
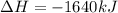
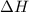 for the reaction comes out to be negative.
for the reaction comes out to be negative.

