
Chemistry, 10.04.2020 05:16 kragland4752
Calculate the volume of 0.500 M HCOOH and 0.500 M HCOONa required to prepare 0.100 L of pH = 4.25 buffer with a buffer
strength of 0.100 M. The pk, of HCOOH is 3.75.
нсоон:
HCOONa:

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Calculate the volume of 0.500 M HCOOH and 0.500 M HCOONa required to prepare 0.100 L of pH = 4.25 bu...
Questions

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Physics, 17.02.2021 23:30


Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Chemistry, 17.02.2021 23:30

Biology, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

History, 17.02.2021 23:30




English, 17.02.2021 23:30


English, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

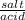
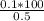 = 20 is the second equation.
= 20 is the second equation.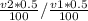 = 5.62
= 5.62

