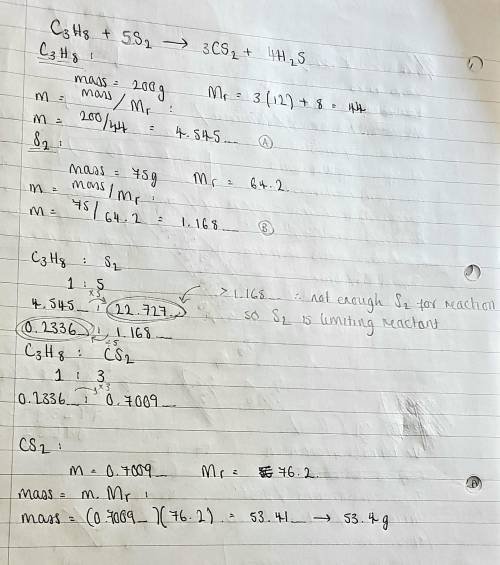Chemistry, 10.04.2020 16:30 NikkiZoeller
Really need help!You have arrived on a planet where S2, not O2, is the gas used as fuel in combustion
reactions. When gaseous propane (C3H8) is burned in S2, H2S and carbon disulfide
gases are produced. If 200.0 grams of propane and 75.0 grams of S2 were reacted,
what is the theoretical yield of carbon disulfide. Include
the balanced equations please

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
Really need help!You have arrived on a planet where S2, not O2, is the gas used as fuel in combustio...
Questions

Mathematics, 13.01.2020 15:31




History, 13.01.2020 15:31

English, 13.01.2020 15:31

Biology, 13.01.2020 15:31



Mathematics, 13.01.2020 15:31




Mathematics, 13.01.2020 15:31

History, 13.01.2020 15:31



Mathematics, 13.01.2020 15:31

Biology, 13.01.2020 15:31