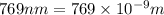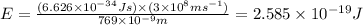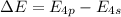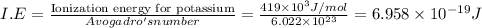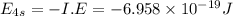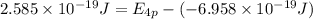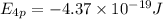Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

You know the right answer?
The ionization energy for potassium 419 kj/mol. the wavelength of light emitted when an excited k at...
Questions

Biology, 31.01.2020 19:01

English, 31.01.2020 19:01



Mathematics, 31.01.2020 19:01


Biology, 31.01.2020 19:01





Computers and Technology, 31.01.2020 19:01

History, 31.01.2020 19:01

History, 31.01.2020 19:01


Mathematics, 31.01.2020 19:01


Mathematics, 31.01.2020 19:01

Arts, 31.01.2020 19:01

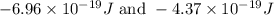
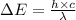
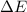 = change in energy
= change in energy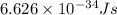
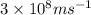
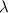 =
=