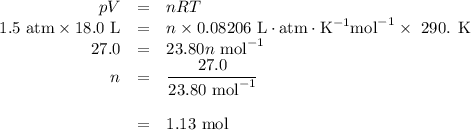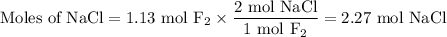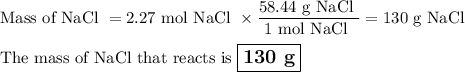I need this answered ASAP!
Part 1. A chemist reacted 18.0 liters of F2 gas with NaCl in the la...

Chemistry, 11.04.2020 00:53 kuehnkeegan
I need this answered ASAP!
Part 1. A chemist reacted 18.0 liters of F2 gas with NaCl in the laboratory to form Cl2 gas and NaF. Use the ideal gas law equation to determine the mass of NaCl that reacted with F2 at 290. K and 1.5 atm. F2 + 2NaCl → Cl2 + 2NaF
Part 2. Explain how you would determine the mass of sodium chloride that can react with the same volume of fluorine gas at STP.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

You know the right answer?
Questions







Mathematics, 23.12.2020 01:00

Mathematics, 23.12.2020 01:00

Social Studies, 23.12.2020 01:00


Mathematics, 23.12.2020 01:00


Mathematics, 23.12.2020 01:00

History, 23.12.2020 01:00

Health, 23.12.2020 01:00

English, 23.12.2020 01:00

Mathematics, 23.12.2020 01:00


Mathematics, 23.12.2020 01:00