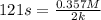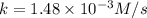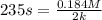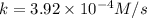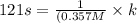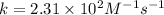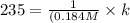Chemistry, 11.04.2020 03:07 reneebrown017
A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.357 M. The same reactant decomposes with a half-life of 235 s when its initial concentration is 0.184 M.
What is the value and unit of the rate constant for this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 23.06.2019 10:00
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1
You know the right answer?
A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.357 M...
Questions

English, 18.03.2021 03:20

Social Studies, 18.03.2021 03:20


English, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Engineering, 18.03.2021 03:20

Social Studies, 18.03.2021 03:20

English, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20


Biology, 18.03.2021 03:20


Health, 18.03.2021 03:20


History, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

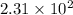 and
and 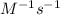 respectively.
respectively.![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0594/9562/b5b11.png)
![t_{1/2}=\frac{1}{[A_o]k}](/tpl/images/0594/9562/15d11.png)