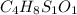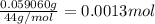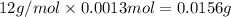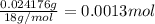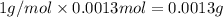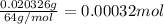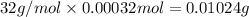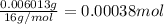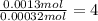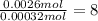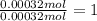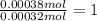A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 59.060 mg of carbon dioxide and 24.176 mg of water. In another experiment, 47.029 mg of the compound is reacted with excess oxygen to produce 20.326 mg of sulfur dioxide. Add subscripts below to correctly identify the empirical formula of this compound (use this order of elements: CHSO)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
A 33.153 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put i...
Questions

Mathematics, 30.04.2021 19:00

Mathematics, 30.04.2021 19:00

English, 30.04.2021 19:00



History, 30.04.2021 19:00


Mathematics, 30.04.2021 19:00

Mathematics, 30.04.2021 19:00



Arts, 30.04.2021 19:00

Physics, 30.04.2021 19:00

Mathematics, 30.04.2021 19:00


Mathematics, 30.04.2021 19:00



Biology, 30.04.2021 19:00