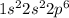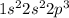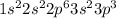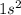Chemistry, 11.04.2020 04:03 browneyedbaby20
Which of the following electron configurations represents an excited state of the indicated atom? Group of answer choices
a. Na: 1s2 2s2 2p6 3s2 3p2 3s1
b. Ne: 1s2 2s2 2p6
c. N: 1s2 2s2 2p3
d. P: 1s2 2s2 2p6 3s2 3p2 4s1
e. He: 1s2

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
Which of the following electron configurations represents an excited state of the indicated atom? Gr...
Questions



Mathematics, 07.03.2020 05:12



Chemistry, 07.03.2020 05:12







Mathematics, 07.03.2020 05:12





Mathematics, 07.03.2020 05:12

Mathematics, 07.03.2020 05:12