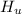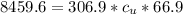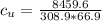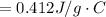Chemistry, 11.04.2020 04:47 nihadsalim10
179.1 g of water is in a Styrofoam calorimeter of negligible heat capacity. The initial T of the water is 16.1oC. After 306.9 g of an unknown compound at 94.3oC is added, the equilibrium T is 27.4oC.
What is the specific heat of the unknown compound in J/(goC)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
179.1 g of water is in a Styrofoam calorimeter of negligible heat capacity. The initial T of the wat...
Questions


Social Studies, 07.07.2019 21:30




Mathematics, 07.07.2019 21:30

Computers and Technology, 07.07.2019 21:30


English, 07.07.2019 21:30

History, 07.07.2019 21:30

History, 07.07.2019 21:30

Advanced Placement (AP), 07.07.2019 21:30

Mathematics, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30



Mathematics, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30

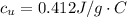
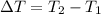
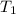 and 27.4°C for
and 27.4°C for 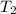
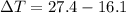
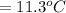
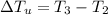
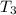
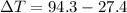
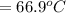
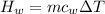
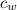 (specific heat of water)
(specific heat of water)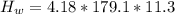
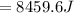
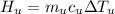
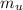 , 8459.6J for
, 8459.6J for