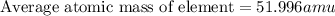Chemistry, 16.10.2019 03:00 22swittman
There are four naturally occurring isotopes of the element chromium. the relative abundance of each is 50cr - 4.345% 49.946044 amu 52cr - 83.789% 51.940508 amu 53cr - 9.501% 52.940649 amu 54cr - 2.365% 53.93880 amu find the average atomic mass of chromium. a. 52.061 amu b. 52.978 amu c. 51.996 amu d. 53.2503 amu

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
There are four naturally occurring isotopes of the element chromium. the relative abundance of each...
Questions

Mathematics, 29.10.2019 13:31

Biology, 29.10.2019 13:31

Chemistry, 29.10.2019 13:31

Mathematics, 29.10.2019 13:31

English, 29.10.2019 13:31


Business, 29.10.2019 13:31

Geography, 29.10.2019 13:31

Mathematics, 29.10.2019 13:31

Mathematics, 29.10.2019 13:31

History, 29.10.2019 13:31


Mathematics, 29.10.2019 13:31



Physics, 29.10.2019 13:31

English, 29.10.2019 13:31

History, 29.10.2019 13:31

History, 29.10.2019 13:31

Mathematics, 29.10.2019 13:31

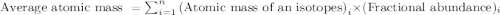
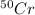 = 49.946044 amu
= 49.946044 amu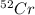 = 51.940508 amu
= 51.940508 amu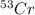 = 52.940649 amu
= 52.940649 amu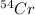 = 53.93880 amu
= 53.93880 amu![\text{Average atomic mass of element}=\sum[(49.946044\times 0.04345)+(51.940508\times 0.83789)+(52.940649\times 0.09501)+(53.93880\times 0.02365)]](/tpl/images/0323/8337/e3693.png)