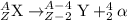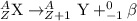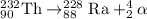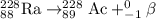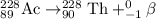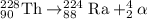The parent nuclide of the thorium decay series is 23290 Th. The first four decays are as follows: alpha emission, beta emission, beta emission, and alpha emission. Use the daughter isotope from the previous equation a the parent isotope for the new equation. Write the 4 nuclear equations for this series of emissions

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
The parent nuclide of the thorium decay series is 23290 Th. The first four decays are as follows: al...
Questions





Mathematics, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40

Arts, 20.02.2021 01:40

Spanish, 20.02.2021 01:40

English, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40




Mathematics, 20.02.2021 01:40

English, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40

Mathematics, 20.02.2021 01:40