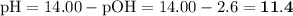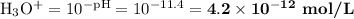Chemistry, 13.04.2020 01:10 alejandro8212003
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2

Chemistry, 23.06.2019 10:30
Chemical bonds result from the interaction of the from two or more atoms. a. protons b. electrons c. neutrons d. nuclei
Answers: 2
You know the right answer?
Ammonia has a Kb of 1.8 × 10−5. Find [H3O+], [OH−], pH, and pOH for a 0.310 M ammonia solution....
Questions


Mathematics, 30.01.2021 23:10

English, 30.01.2021 23:10


Geography, 30.01.2021 23:10

Spanish, 30.01.2021 23:10


Mathematics, 30.01.2021 23:10

Mathematics, 30.01.2021 23:10





Mathematics, 30.01.2021 23:10






Mathematics, 30.01.2021 23:10

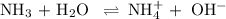
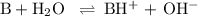
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.100 - x} = 1.8 \times 10^{-5}](/tpl/images/0596/0459/4767e.png)
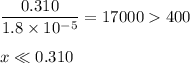
![\dfrac{x^{2}}{0.310} = 1.8 \times 10^{-5}\\\\x^{2} = 0.310 \times 1.8 \times 10^{-5}\\x^{2} = 5.58 \times 10^{-6}\\x = \sqrt{5.58 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.4 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0596/0459/7dcd2.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.4 \times 10^{-3}) = \mathbf{2.6}](/tpl/images/0596/0459/b8dea.png)