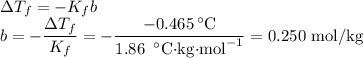Chemistry, 13.04.2020 01:29 Theresab2021
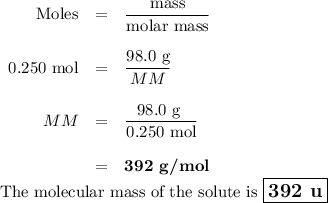
Suppose that 98.0g of a non electrolyte is dissolved in 1.00kg of water. The freezing point of this solution is found to be -0.465. What is the molecular mass of the solute?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Suppose that 98.0g of a non electrolyte is dissolved in 1.00kg of water. The freezing point of this...
Questions

English, 16.10.2020 18:01



Physics, 16.10.2020 18:01


English, 16.10.2020 18:01

Advanced Placement (AP), 16.10.2020 18:01


Social Studies, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Social Studies, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

English, 16.10.2020 18:01

Arts, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01