The above reaction was used to fuel the rockets in the Apollo mission landing module.
A)...

Chemistry, 13.04.2020 07:39 sierrabuckner397
The above reaction was used to fuel the rockets in the Apollo mission landing module.
A) Is this reaction endothermic or exothermic?
B) How many grams of N2H4 must be reacted with an excess of N2O4 to produce 775 kJ of energy?
C) How many kJ of energy are produced when 6.25 g of N2O4 reacts with an excess of N2H4?
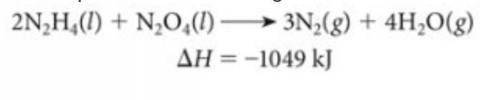

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Questions

Mathematics, 14.01.2021 03:30



Arts, 14.01.2021 03:30

Mathematics, 14.01.2021 03:30




English, 14.01.2021 03:30

English, 14.01.2021 03:30


English, 14.01.2021 03:30

Computers and Technology, 14.01.2021 03:30


Mathematics, 14.01.2021 03:30

Mathematics, 14.01.2021 03:30


Mathematics, 14.01.2021 03:30




