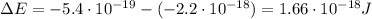Chemistry, 13.04.2020 11:27 jrfranckowiak
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
and has a wavelength of 532 nm.
a) What is the frequency of this light?
b) What is the energy in Joules of one photon of this light?
c) What is the energy in Joules of one mole of photons of this light?
d) What is the orbital energy in Joules of an electron in the ground state (n=1) in a hydrogen atom
according to the Bohr model?
e) What is the orbital energy in Joules of an electron in the n=2 state in a hydrogen atom according to
the Bohr model?


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3
You know the right answer?
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
Questions



Biology, 28.01.2021 20:10


Health, 28.01.2021 20:10


English, 28.01.2021 20:10


Mathematics, 28.01.2021 20:10


Mathematics, 28.01.2021 20:10

Mathematics, 28.01.2021 20:10



Physics, 28.01.2021 20:10

Mathematics, 28.01.2021 20:10

Social Studies, 28.01.2021 20:10

Mathematics, 28.01.2021 20:10

Biology, 28.01.2021 20:10

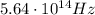
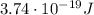
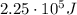
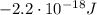
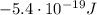
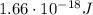
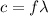
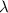 is the wavelength
is the wavelength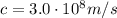 is the speed of light
is the speed of light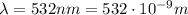 is the wavelength of the green light emitted by the laser
is the wavelength of the green light emitted by the laser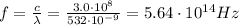
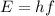
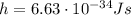 is the Planck's constant
is the Planck's constant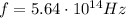 is the frequency of the photon
is the frequency of the photon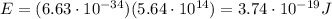
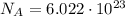
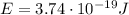
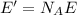
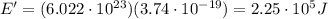
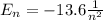 [eV]
[eV]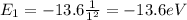
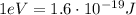
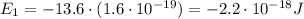
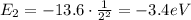
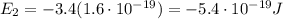
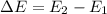
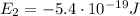 is the energy of orbital n=2
is the energy of orbital n=2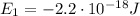 is the energy of orbital n=1
is the energy of orbital n=1