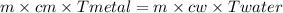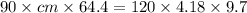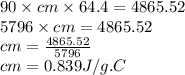Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
A 90.0 g piece of metal, initially at 98.6°C, is placed into 120.0 g of
water initially at 24....
water initially at 24....
Questions

Physics, 16.01.2020 11:31

Mathematics, 16.01.2020 11:31

History, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31

English, 16.01.2020 11:31

Mathematics, 16.01.2020 11:31


Chemistry, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31

Mathematics, 16.01.2020 11:31




Mathematics, 16.01.2020 11:31


History, 16.01.2020 11:31

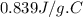 .
.