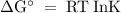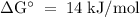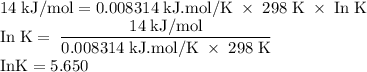Chemistry, 13.04.2020 22:28 ksoodagoat
The standard biological reaction Gibbs energy for the removal of the phosphate group from adenosine monophosphate is 14 kJ mol-1 at 298 K. What is the value of the thermodynamic standard reaction Gibbs energy?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
The standard biological reaction Gibbs energy for the removal of the phosphate group from adenosine...
Questions








English, 18.12.2019 18:31



English, 18.12.2019 18:31

Engineering, 18.12.2019 18:31


Mathematics, 18.12.2019 18:31

English, 18.12.2019 18:31

Mathematics, 18.12.2019 18:31




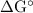 ) has been given by:
) has been given by: