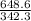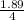Chemistry, 13.04.2020 22:49 20warriorsoul14
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of the solution?
The chemical formula for sucrose is C12H22O11 , and the molar mass is 342.3 g/mol. mol/L

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2
You know the right answer?
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of...
Questions


History, 31.01.2020 05:00





English, 31.01.2020 05:00

Mathematics, 31.01.2020 05:00



Mathematics, 31.01.2020 05:00

History, 31.01.2020 05:00



Mathematics, 31.01.2020 05:00

Social Studies, 31.01.2020 05:00



Mathematics, 31.01.2020 05:00

Mathematics, 31.01.2020 05:00

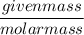
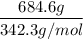
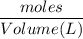

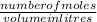 equation 1
equation 1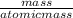 equation 2
equation 2