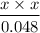Chemistry, 14.04.2020 04:27 lizdominguez101
Determine the pH of a 0.048 M hypochlorous acid (HClO) solution. Hypochlorous acid is a weak acid (Ka = 4.0 ✕ 10−8 M).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

You know the right answer?
Determine the pH of a 0.048 M hypochlorous acid (HClO) solution. Hypochlorous acid is a weak acid (K...
Questions

Advanced Placement (AP), 13.10.2020 02:01


History, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01



Mathematics, 13.10.2020 02:01


Physics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

English, 13.10.2020 02:01


English, 13.10.2020 02:01


Health, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

![$\frac{[H^{+}] [ClO^{-}] }{[HClO]}](/tpl/images/0597/7831/56a50.png)