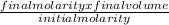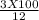Chemistry, 14.04.2020 06:12 jessicaa2350
In order to complete a lab, your teacher needs a 3.0 M solution of sulfuric acid, but only has a 12.0 M stock solution of sulfuric acid in the chemical store room. Calculate and describe the steps the teacher needs to take in order to make 100 mL of the 3.0 M solution of sulfuric acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

You know the right answer?
In order to complete a lab, your teacher needs a 3.0 M solution of sulfuric acid, but only has a 12....
Questions

Social Studies, 22.02.2021 20:20

Mathematics, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20

Spanish, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20

Mathematics, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20


English, 22.02.2021 20:20



History, 22.02.2021 20:20


Mathematics, 22.02.2021 20:20

Spanish, 22.02.2021 20:20