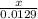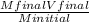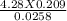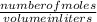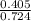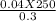Chemistry, 14.04.2020 07:44 aide1234564
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (aq) -->
2. How many L of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO3)2 according to the balanced chemical reaction:
2Cu(NO₃)₂(aq) + 4KI(aq) → 2CuI(aq) + I₂(s) + 4KNO₃(aq)
3.What volume in L of a 0.724 M Nal solution contains0.405 mol of Nal?
4. How many mL of 0.300 M NaF would be required to make a 0.0400 M solution of NaF when diluted to 250.0 mL with water?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1
You know the right answer?
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (...
H2SO4 (aq) + Sr(OH)2 (...
Questions

Mathematics, 08.06.2021 20:20

Mathematics, 08.06.2021 20:20


Biology, 08.06.2021 20:30



Mathematics, 08.06.2021 20:30



Mathematics, 08.06.2021 20:30

Advanced Placement (AP), 08.06.2021 20:30

Mathematics, 08.06.2021 20:30

Mathematics, 08.06.2021 20:30


Mathematics, 08.06.2021 20:30

Mathematics, 08.06.2021 20:30

Mathematics, 08.06.2021 20:30


Mathematics, 08.06.2021 20:30

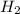 S
S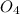 + Sr(OH)
+ Sr(OH) ⇒ SrSO4 + 2 H2O is the balanced reaction.
⇒ SrSO4 + 2 H2O is the balanced reaction.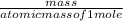
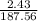
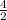 =
=