
Chemistry, 14.04.2020 16:12 buiratsamah
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393.5 kJ/mol ) and water (H2 O, Hf = –241.82 kJ/mol) according to the equation below. What is the enthalpy of combustion (per mole) of C4H10 (g)? Use .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1


Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Butane (C4 H10(g), Hf = –125.6 kJ/mol) reacts with oxygen to produce carbon dioxide (CO2 , Hf = –393...
Questions

Mathematics, 27.02.2021 07:30


Mathematics, 27.02.2021 07:30

Biology, 27.02.2021 07:30

English, 27.02.2021 07:30

Mathematics, 27.02.2021 07:30

Mathematics, 27.02.2021 07:30

Mathematics, 27.02.2021 07:30

Mathematics, 27.02.2021 07:30

Mathematics, 27.02.2021 07:30

Chemistry, 27.02.2021 07:30









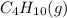 is -2657.5 kJ
is -2657.5 kJ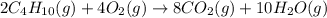
![\Delta H^o_{rxn}=[(8\times \Delta H^o_f_{CO_2(g)})+(10\times \Delta H^o_f_{H_2O(g)})]-[(1\times \Delta H^o_f_{C_4H_{10}(g)})+(4\times \Delta H^o_f_{O_2(g)})]](/tpl/images/0598/2199/66d33.png)
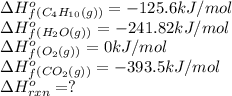
![\Delta H^o_{rxn}=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.6)+(4\times 0)]\\\\\Delta H^o_{rxn}=-5315kJ](/tpl/images/0598/2199/af1b6.png)


