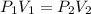Chemistry, 14.04.2020 16:33 lindasuebairdoyjpf7
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 80.0 atm , what was the volume of the sample

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3
You know the right answer?
A sample of an ideal gas at 1.00 atm and a volume of 1.87 L was placed in a weighted balloon and dro...
Questions




Mathematics, 01.01.2021 23:10

Chemistry, 01.01.2021 23:10


Chemistry, 01.01.2021 23:10



Chemistry, 01.01.2021 23:10

Mathematics, 01.01.2021 23:10



Spanish, 01.01.2021 23:10

Mathematics, 01.01.2021 23:10





 and
and  are the initial gas parameter before dropping into the ocean
are the initial gas parameter before dropping into the ocean and
and  are the final gas parameter after dropping into the ocean
are the final gas parameter after dropping into the ocean