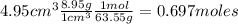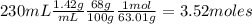Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. What volume (in L) of nitrogen dioxide is formed at 735 torr and 28.2°C by reacting 4.95 cm3 of copper (d = 8.95 g/cm3 ) with 230.0 mL of nitric acid (d = 1.42 g/cm3 , 68.0% HNO3 by mass)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Arts, 09.04.2020 03:56

Mathematics, 09.04.2020 03:56

English, 09.04.2020 03:56

Mathematics, 09.04.2020 03:56





Biology, 09.04.2020 03:57







Mathematics, 09.04.2020 03:57


Mathematics, 09.04.2020 03:57

History, 09.04.2020 03:57