
Chemistry, 14.04.2020 18:05 rileyeddins1010
What is the % yield of the reaction making 1.6 g of acetyl salicylic acid from 5.7 g of salicylic acid, 9.9 mL of acetic anhydride (density 1.082), and 10 drops 85 % weight phosphoric acid (density 1.685)? Use the molecular weights that you have already calculated. Give two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2


Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
What is the % yield of the reaction making 1.6 g of acetyl salicylic acid from 5.7 g of salicylic ac...
Questions


History, 31.10.2019 18:31

Mathematics, 31.10.2019 18:31







English, 31.10.2019 18:31

Arts, 31.10.2019 18:31

English, 31.10.2019 18:31



Business, 31.10.2019 18:31


Biology, 31.10.2019 18:31

History, 31.10.2019 18:31


Chemistry, 31.10.2019 18:31

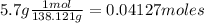
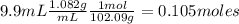
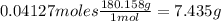
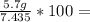 76.7%
76.7%

