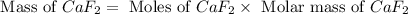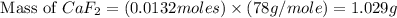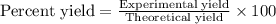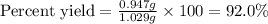Chemistry, 14.04.2020 18:07 Gabriella0000
A 1.103 g sample of sodium fluoride is dissolved in water, and then a precipitate of calcium fluoride is produced by adding a calcium nitrate solution. If the dried calcium fluoride precipitate has a mass of 0.947 g, what is the percent yield?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2


Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
A 1.103 g sample of sodium fluoride is dissolved in water, and then a precipitate of calcium fluorid...
Questions

Mathematics, 05.07.2019 18:30

Geography, 05.07.2019 18:30

Mathematics, 05.07.2019 18:30

Biology, 05.07.2019 18:30




Health, 05.07.2019 18:30


Mathematics, 05.07.2019 18:30

History, 05.07.2019 18:30

Biology, 05.07.2019 18:30

Mathematics, 05.07.2019 18:30

Computers and Technology, 05.07.2019 18:30

Business, 05.07.2019 18:30

History, 05.07.2019 18:30


Mathematics, 05.07.2019 18:30

Mathematics, 05.07.2019 18:30

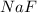 = 1.103 g
= 1.103 g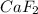 = 78 g/mol
= 78 g/mol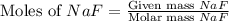
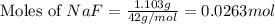
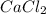
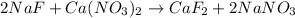
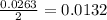 mole of
mole of