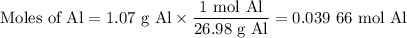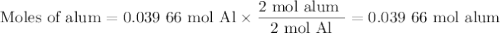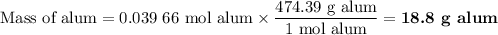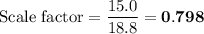Chemistry, 14.04.2020 18:53 sierravick123owr441
Scaled Synthesis of Alum. Show your calculations for:a. the experimental scaling factor giving rise to a 15.0 g theoretical yield;b. the corrected volumes of KOH and H2SO4; andc. the theoretical yield of alum based on the actual amount of Al used. Make sure you carefully show each step for these calculations.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
Scaled Synthesis of Alum. Show your calculations for:a. the experimental scaling factor giving rise...
Questions

History, 28.12.2019 08:31

Mathematics, 28.12.2019 08:31

Mathematics, 28.12.2019 08:31


Mathematics, 28.12.2019 08:31



English, 28.12.2019 08:31


Mathematics, 28.12.2019 08:31


History, 28.12.2019 08:31





Social Studies, 28.12.2019 08:31



Physics, 28.12.2019 08:31