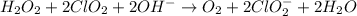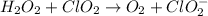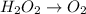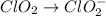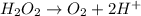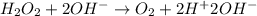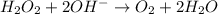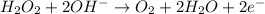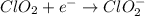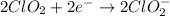Chemistry, 14.04.2020 20:14 cadereymer24
Given the partial equation: H2O2 + ClO2 → O2 + ClO2−, balance the reaction in basic solution using the half-reaction method and fill in the coefficients. The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients. H2O2 + ClO2 + → O2 + ClO2− +

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Given the partial equation: H2O2 + ClO2 → O2 + ClO2−, balance the reaction in basic solution using t...
Questions


History, 25.01.2021 22:00

Mathematics, 25.01.2021 22:00



Mathematics, 25.01.2021 22:00



Arts, 25.01.2021 22:00


English, 25.01.2021 22:00


Mathematics, 25.01.2021 22:00


Mathematics, 25.01.2021 22:00



Social Studies, 25.01.2021 22:00


English, 25.01.2021 22:00