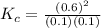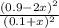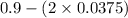Chemistry, 14.04.2020 19:52 KariSupreme
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=[O2]=0.100 M and [ NO ] = 0.600 M . [NO]=0.600 M. N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) N2(g)+O2(g)↽−−⇀2NO(g) If more NO NO is added, bringing its concentration to 0.900 M, 0.900 M, what will the final concentration of NO NO be after equilibrium is re‑established?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
At equilibrium, the concentrations in this system were found to be [ N 2 ] = [ O 2 ] = 0.100 M [N2]=...
Questions

Mathematics, 17.12.2019 22:31

History, 17.12.2019 22:31

Chemistry, 17.12.2019 22:31

History, 17.12.2019 22:31

Mathematics, 17.12.2019 22:31

Mathematics, 17.12.2019 22:31


History, 17.12.2019 22:31


History, 17.12.2019 22:31



Social Studies, 17.12.2019 22:31

Mathematics, 17.12.2019 22:31

History, 17.12.2019 22:31

Health, 17.12.2019 22:31



Mathematics, 17.12.2019 22:31

Arts, 17.12.2019 22:31

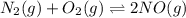
![K_{c} = \frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0599/0103/a7a07.png)
![[N_{2}] = [O_{2}]](/tpl/images/0599/0103/c9b74.png) = 0.1 M and [NO] = 0.6 M
= 0.1 M and [NO] = 0.6 M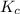 is as follows.
is as follows.