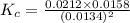Chemistry, 14.04.2020 20:01 dontworry48
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles of CH4, and 0.240 moles of CCl4 are at equilibrium in a 15.2 L container at 477 K, the value of the equilibrium constant, Kc, is

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1
You know the right answer?
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles...
Questions




Mathematics, 22.03.2021 17:10


Mathematics, 22.03.2021 17:10





Chemistry, 22.03.2021 17:10


English, 22.03.2021 17:10







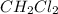 at equilibrium= 0.203 mole
at equilibrium= 0.203 mole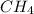 at equilibrium = 0.323 mole
at equilibrium = 0.323 mole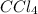 at equilibrium = 0.240mole
at equilibrium = 0.240mole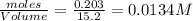
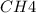 =
= 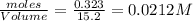
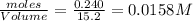
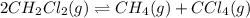
![K_c=\frac{[CH_4]\times [CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0599/0518/28fa5.png)