
Chemistry, 14.04.2020 21:12 larapoghosyan91
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-calming agent. What is the hydronium ion concentration (in M) in a 0.737 M solution of HC5H9O2? (Assume Kw = 1.01 ✕ 10−14.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-c...
Questions




Mathematics, 09.07.2021 21:40

Mathematics, 09.07.2021 21:40

History, 09.07.2021 21:40














Mathematics, 09.07.2021 21:40

![[H^+]=0.00332M](/tpl/images/0599/2796/eac47.png)
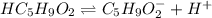
![Ka=\frac{[H^+][C_5H_9O_2^-]}{[HC_5H_9O_2]}](/tpl/images/0599/2796/6b49e.png)
 due to dissociation, it becomes:
due to dissociation, it becomes: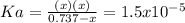
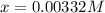
![[H^+]=x=0.00332M](/tpl/images/0599/2796/13d16.png)


