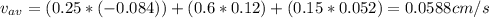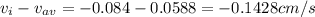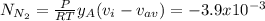A gaseous mixture at 265K and 1.0 atm contains 25 O2 60 N2 and 15 CO2 mole basis The velocities of the components are 0.084 cm s O2 0.120 cm s N2 and 0.052 cm s CO2 Find the N2 diffusion velocity relative to the mole average velocity and the molar diffusional flux of N2

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A gaseous mixture at 265K and 1.0 atm contains 25 O2 60 N2 and 15 CO2 mole basis The velocities of t...
Questions


History, 15.10.2021 08:10

Mathematics, 15.10.2021 08:10

Mathematics, 15.10.2021 08:10

Chemistry, 15.10.2021 08:10

Mathematics, 15.10.2021 08:20

Geography, 15.10.2021 08:20





Biology, 15.10.2021 08:30

Mathematics, 15.10.2021 08:40

History, 15.10.2021 08:40

Mathematics, 15.10.2021 08:40