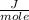Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
The concentration of glucose inside a cell is 0.12 mM. Outside the cell the concentration of glucose...
Questions

Social Studies, 19.03.2020 02:21

Mathematics, 19.03.2020 02:21


History, 19.03.2020 02:21

Mathematics, 19.03.2020 02:21

Mathematics, 19.03.2020 02:21


Physics, 19.03.2020 02:21


Mathematics, 19.03.2020 02:22

Mathematics, 19.03.2020 02:22







Mathematics, 19.03.2020 02:22

Mathematics, 19.03.2020 02:22

Mathematics, 19.03.2020 02:22