

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3

You know the right answer?
Calculate the concentration of H3O in a solution that contains 5.5 × 10-5 M OH at 25°C. Identify the...
Questions



Computers and Technology, 22.04.2020 16:30


Biology, 22.04.2020 16:30






Biology, 22.04.2020 16:30

Biology, 22.04.2020 16:30






History, 22.04.2020 16:30

Biology, 22.04.2020 16:30

Social Studies, 22.04.2020 16:30

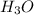 is 1.8×10⁻¹⁰ M and it is basic in nature.
is 1.8×10⁻¹⁰ M and it is basic in nature.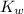 =
= 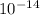 (at 25°C)
(at 25°C) ]
]![\Rightarrow [H_3O^+]=\frac{10^{-14}}{5.5\times 10^{-5}}](/tpl/images/0599/5597/3fdf6.png)


